The most commonly used immuno- or antibody-based test for GMO detection is the strip test (also called a lateral flow device or dipstick). Strip tests are thin strips comprised of a nitrocellulose membrane covered by a sample pad on one end and a wicking pad on the other end.
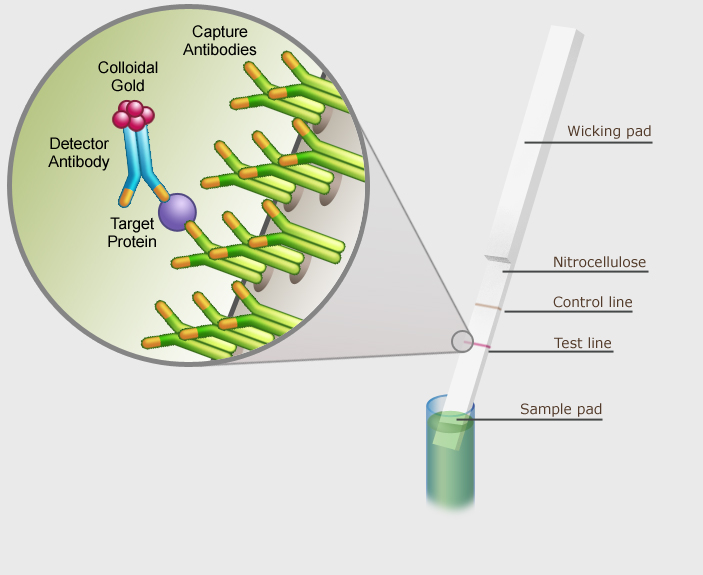
The sample pad is submersed in a solution of homogenized test sample. The solution wicks up the nitrocellulose membrane on the strip, causing the fluid to pass over an area containing an excess of gold-labeled antibody specific to the GMO protein being tested. If that particular GMO protein is present in the sample then it will specifically bind to the gold-labeled antibody, and the antibody-protein complex will continue moving up the strip with the flow of fluid.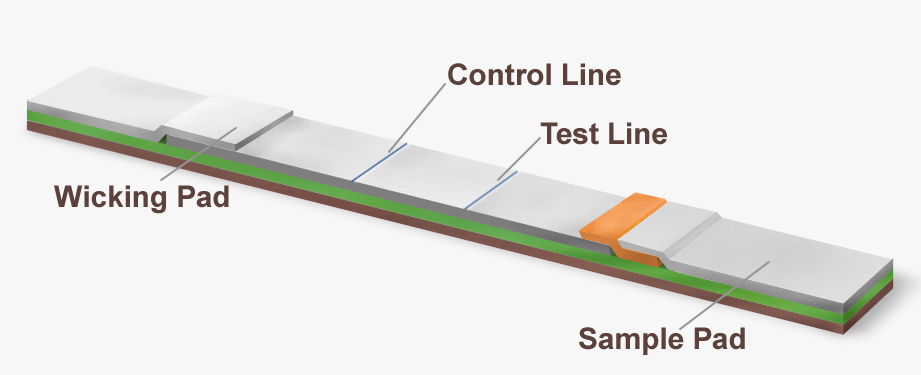

The sample fluid then passes over two additional areas on the strip, a test line and a control line. The test line contains a second antibody which is specific for the GMO protein being tested. When the gold-labeled antibodies with bound GMO proteins pass over the test line, the antibody-protein complex forms a sandwich with the immobilized second capture antibody. This results in formation of a visible line on the strip indicating that that particular GMO protein is present in the sample.
Excess unbound gold-labeled antibody continues to flow up the strip and passes over the control line which contains a third immobilized antibody specific for the gold-labeled antibody. When the excess gold-labeled antibodies bind to this immobilized capture antibody, it produces a second visible line that serves as an internal control to ensure that gold-labeled antibodies have passed through all three check points.
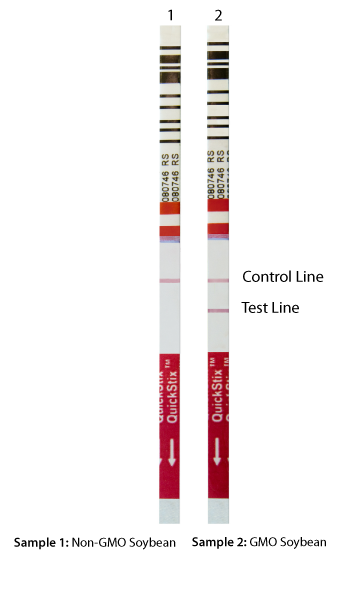 The sample is considered positive for the GMO protein of interest if the test and control lines are visible whereas the sample is considered negative for the GMO protein if only the control line is visible. Absence of both lines indicates that the test is invalid and should be repeated.
The sample is considered positive for the GMO protein of interest if the test and control lines are visible whereas the sample is considered negative for the GMO protein if only the control line is visible. Absence of both lines indicates that the test is invalid and should be repeated.
The strip test as a GMO detection method has an important place in the production system. The assay is rapid, is appropriate for qualitative or semi-quantitative detection of GMO proteins, and can be performed in the field. Strip tests are therefore applicable for an initial screen of seed/grain at, for example, the elevator, or from the truck upon arrival at the processing plant.
Although the speed and convenience of antibody-based tests offer substantial utility, a crucial limitation of these tests is that not all GMO transgenes code for a protein. Therefore, antibody-based tests are not available for all commercialized GMOs. Another limitation of strip tests is that the sensitivity is not as great as that of PCR tests. For example, the limit of detection of strip tests is usually within the 0.1 – 1% range, whereas the PCR (DNA) test method is more sensitive, with a limit of detection of 0.01%.
Antibody-based tests are also not appropriate for heat or chemically processed products (e.g., soy protein isolate, lecithin, etc.) in which the protein is denatured resulting in destruction of the antibody binding site. The protein in processed products is much more susceptible to degradation than is the DNA; therefore while protein-based analysis is not appropriate for GMO testing of processed products, DNA-based PCR analysis may be used for GMO testing of many processed sample types. Finally, there may be differences in GM protein levels between different commercial GM cultivars as well as between different parts of the GM plant. All of these factors need to be taken into consideration when considering antibody-based tests. However, for seed/grain, in which the protein is intact, GMO strip tests provide an important and rapid on-site initial screening method.
